Streamlining Success: How Veeva Vault QMS Transforms Change Control and Document Management
- contact58607
- Oct 8, 2024
- 2 min read
Updated: Dec 23, 2024
Introduction
Veeva Vault QMS is a comprehensive quality management system designed to streamline and automate various quality-related processes within life sciences organizations. One of its key functionalities is the effective management of change control and document management. This article delves into how Veeva Vault QMS can be leveraged to streamline these critical processes.
Change Control Management
Change control is a fundamental aspect of quality management, ensuring that any modifications to processes, procedures, or documentation are implemented in a controlled and documented manner. Veeva Vault QMS offers a robust change control module that simplifies this process:

Document Management
Effective document management is essential for maintaining compliance and ensuring that all relevant information is readily accessible. Veeva Vault QMS provides a centralized repository for storing and managing documents:

Integration with Other Modules
Veeva Vault QMS seamlessly integrates with other modules within the Veeva Vault platform, such as Vault Clinical Suite and Vault Commercial Suite. This integration ensures that quality data and processes are aligned with other critical business functions, enhancing overall efficiency and compliance.
Benefits of Using Veeva Vault QMS for Change Control and Document Management
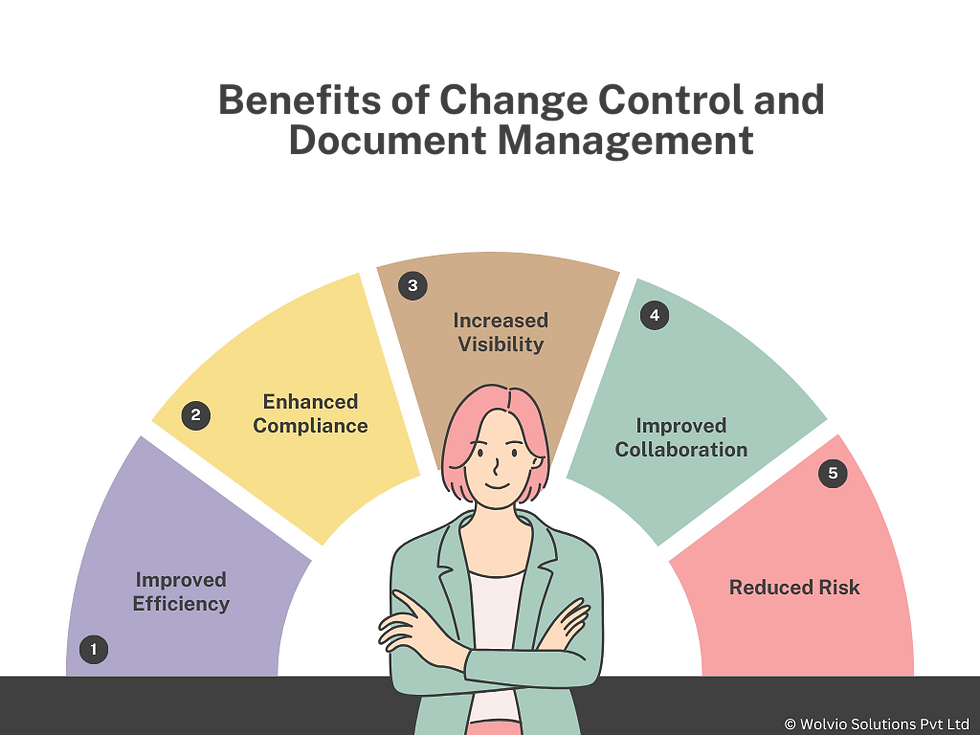
Improved Efficiency: Streamlined workflows and automation reduce manual effort and accelerate processes.
Enhanced Compliance: Veeva Vault QMS helps organizations meet regulatory requirements and maintain a strong quality management system.
Increased Visibility: Centralized document management and change control provide better visibility into processes and documentation.
Improved Collaboration: The system facilitates collaboration among teams, ensuring that all stakeholders are aligned and informed.
Reduced Risk: Effective change control and document management help mitigate risks associated with non-compliance and errors.
Conclusion
By leveraging Veeva Vault QMS for change control and document management, life sciences organizations can establish a robust and efficient quality management system that supports compliance, improves operational efficiency, and drives innovation.
The system's capabilities, including automation, integration, and advanced features, enable organizations to streamline processes, reduce errors, and ensure that quality standards are consistently met. If you are looking to enhance your organization's change control and document management capabilities, consider implementing Veeva Vault QMS. To learn more about how Veeva Vault QMS can benefit your organization, contact us today for a consultation.
#VeevaVault #QualityManagement #ChangeControl #DocumentManagement #LifeSciences #Compliance #RegulatoryCompliance #ClinicalTrials #DigitalTransformation #VeevaSolutions #WolvioSolutions


Comments